How to prepare the surface of the new generation titanium implants?
2022.05.27 9:13 - Piotr Spinalski
 Titanium-based materials are very often used in biomaterials engineering for the production of implants used in medicine. Due to the working conditions of such materials, their physicochemical properties, especially on the surface, must be precisely controlled already at the manufacturing stage. For the first time, scientists from the Materials Research Laboratory and the Center of Excellence NOMATEN NCBJ used the Raman imaging research technique to determine the spatial distribution and quantitative proportion of individual phases in titanium oxide.
Titanium-based materials are very often used in biomaterials engineering for the production of implants used in medicine. Due to the working conditions of such materials, their physicochemical properties, especially on the surface, must be precisely controlled already at the manufacturing stage. For the first time, scientists from the Materials Research Laboratory and the Center of Excellence NOMATEN NCBJ used the Raman imaging research technique to determine the spatial distribution and quantitative proportion of individual phases in titanium oxide.
Currently, titanium and its alloys occupy a significant part of the biomaterial production market, especially in orthopedic and dental implantology. This is due to two key factors related to the properties of titanium. The first is biocompatibility, which means that the contact of the material with the body’s tissue does not cause a negative reaction, such as metallosis, as well as allergic and inflammatory reactions, mainly caused by the release of corrosion products into the body. The second valuable feature of titanium in terms of biomedical applications are its unique mechanical properties, similar to human bone tissue. The aforementioned biocompatibility of titanium and its alloys is related to, among other things, the ability of this material to passivate on the surface. On titanium substrates, a layer of titanium oxide TiO2 is formed in two polymorphs: rutile and anatase. The passivation process increases the corrosion resistance of the material, but its spontaneous nature makes it impossible to control the exact phase composition of the oxide formed. This is definitely disadvantageous from the point of view of designing biomaterials, from which the highest reliability is required, and thus strict control of the composition and physicochemical properties of the surface. Therefore, titanium-based materials are subjected to various surface treatments such as e. g. electrochemical oxidation, physical or chemical vapor deposition (PVD, CVD) or sol-gel. The quality of the obtained titanium oxide layers ultimately determines the final properties of the biomaterial not only in terms of corrosion resistance, but also obtaining functional properties of the surface, e. g. bioactivity. In in-vitro and in-vivo tests, bioactivity of both stable rutile and non-equilibrium anatase Was demonstrated, albeit to a lower extent. The bioactivity of the material is a positive and desirable effect, because the material with this property is permanently attached to the bone tissue by means of a chemical bond, positively influencing its reconstruction and vascularization. It also promotes cell multiplication and accelerated bone mineralization. This significantly shortens the patient’s convalescence period and guarantees a long, safe and comfortable use of the implant.
In research conducted by our scientists from the Material Research Laboratory and the NOMATEN Center of Excellence, a detailed surface analysis Was carried out on titanium-based substrates, usually used for biomedical applications, on which TiO2 oxide layers were formed during the oxidation process. The oxidation process Was carried out in two stages, the so-called hybrid technology, which consists of high-temperature diffusion oxidation in a fluidized bed followed by surface oxidation by PVD by magnetron sputtering. In the first process, a diffusion layer and a stable porous rutile are formed, while in the second, a mixture of oxides – rutile and anatase. The final phase composition of the coating and the spatial distribution of individual oxides on the surface depend on the adopted process parameters. „This is crucial, because due to the different affinity and adhesion of reproducing cells to rutile and anatase, the regenerating tissue will behave in a bioactively different manner depending on the phase composition in a given area of the implant surface „– emphasizes PhD. Eng. Jarosław Jasiński from the NCBJ Materials Research Laboratory. „Therefore, it is important to precisely define the spatial distribution as well as the share of rutile and anatase in the obtained layers, especially in the near-surface zone of the biomaterial. The ideal method to define it is Raman imaging. „In the case of the above-mentioned tests, they were performed using the Raman WITec alpha 300 R. microscope located at the NCBJ Materials Research Laboratory. „Due to the high precision and sensitivity of the method, even to subtle structural changes in the tested layers, it is possible to clearly distinguish between the various polymorphs of titanium oxide” – adds PhD. Eng. Magdalena Gawęda from the Center of Excellence NOMATEN NCBJ. „Moreover, the specificity of the device and research method of Raman imaging additionally allows for the preparation of maps showing the spatial distribution of individual oxide phases on the surface of the biomaterial. „On the basis of the obtained maps, it is possible not only to identify the oxides, but also a semi-quantitative analysis, which precisely determines the percentages of rutile and anatase in the layers.
„We have adapted the calculation method proposed earlier to the point measurements of powders only” – explains MSc. Eng. Kinga Suchorab from the Center of Excellence NOMATEN NCBJ, co-author of the work. „Thus, this method Was used for the first time in Raman imaging of thin oxide layers. In this way, we obtained percentage maps of rutile and anatase on titanium substrates. „The analysis of a fairly large surface area of the substrates allowed for precise determination of the uniformity of the coatings made. The obtained results confirmed the assumptions made at the stage of developing the methodology for obtaining hybrid coatings. A thin surface layer of anatase Was obtained on a stable foundation constituting a rutile layer. This Was additionally confirmed by the analysis of the substrate cross-section.
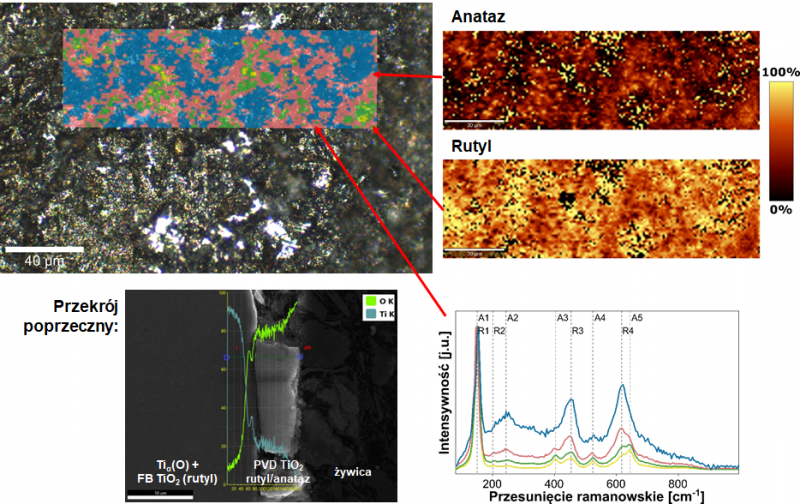
The illustration shows the results of Raman imaging of the titanium oxide surface superimposed on the microscopic image (upper left image), the results of the spatial distribution of rutile and anatase TiO2 polymorphs (upper right image), phase identification based on the position of the Raman bands (spectra in the lower right corner), as well as a scanning electron microscope (SEM) image with EDS linear elemental analysis of titanium and oxygen (lower left image).
The methodology presented by scientists from the Materials Testing Laboratory and the NOMATEN NCBJ Center of Excellence allows for a precise and quick evaluation of the surface of the obtained materials in the context of the dependence of the process parameters, and the final composition and physicochemical properties of the obtained coatings. This can be used in the selection of appropriate parameters for the production of oxide layers in new generation biomaterials. At the same time, the enormous application potential of the research method itself, which is Raman imaging, in surface engineering and material research is visible.
The full study results are available in: J. J. Jasinski, M. Lubas, K. Suchorab, M. Gawęda, L. Kurpaska, M. Brykala, A. Kosinska, M. Sitarz, J. Jagielski, Qualitative and semi-quantitative phase analysis of TiO2 thin layers by Raman imaging, Journal of Molecular Structure, Volume 1260, 2022, 132803, ISSN 0022-2860, https://doi.org/10.1016/j.molstruc.2022.132803