BNCT chemistry
BORON NEUTRON CAPTURE THERAPY
Boron carriers must be low-toxic substances selectively acquired by malignant cells.
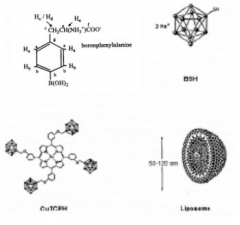
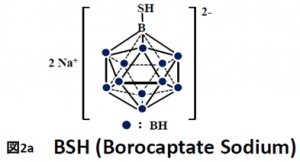
BSH (di-sodium undecahydro-mercapto-closo-dodecacarborate Na2B12H11SH) and BPA (p-boronphenylalanine C9H12BNO4) were the first Boron compounds used in BNCT (see Fig.2). Each of the compound uses another mechanism along which the B-containing molecules enter malignant cells.
BSH passively diffuses from blood stream into malignant cells in brain. The diffusion is possible because malignant cells disrupt the Blood Brain Barrier normally at work within the brain. Simultaneously BSH cannot penetrate healthy cells that do not disturb the barrier. Studies on that mechanism have shown that BSH can selectively penetrate brain cells if the cancer/blood ratio is within the 1:1 to 2:1.6 range [2].
BPA is a boron-labelled amino acid. Such bio-molecules are actively acquired by malignant cells that transport them through their membranes at rates much higher than the rates observed in healthy cells – just to sustain their extraordinarily large growth rates. Concentration of BPA in malignant cells is usually 2-4 times higher than in blood cells and in other healthy cells.
Unfortunately, neither BPA nor BSH penetrate malignant cells uniformly. Therefore distribution of boron – hence distribution of the deposited radiation doses – is not uniform throughout the cells. In consequence some malignant cells survive the therapy. Besides, selectivity is not perfect: some BPA or BSH penetrate also healthy cells. In consequence neutron dose must be limited. To overcome these shortcomings, some attempts have been made to simultaneously administer both BPA and BSH in the hope that the two different penetration mechanisms would supplement each other. Alas, the results were not satisfactory.
Better results were obtained with boron-loaded liposome and boron-loaded porphyrins.
Various liposomes are used as carriers not only in cancer therapy since they are bio-compatible with cell membranes and exhibit low toxicity. Normally blood vessel walls are weakly transparent for liposomes. However, if some inflammation appears within the organism (because of a bacterial/viral infection, or because of a neoplasmatic process), individual cells in blood vessel walls become separated by quite large gaps so that liposomes could leave the vessels and gather at the the inflammation place. All in all, boron-loaded liposomes can accumulate within cancer tumours. The mechanism is referred to as passive accumulation of liposomes.
Porphyrins are aromatic tetrapyrrole compounds. Some of them, for example chlorophyll and hem, naturally occur in plants and animals. They show significant affinity to neoplasmic tissue, low cytotoxicity, and can easily bind large amounts of boron. They are used as carriers also in Photodynamic Therapy.
1. Floberg J., The physics of boron neutron capture therapy: an emerging and innovative treatment for glioblastoma and melanoma, http://digitalcommons.carleton.edu/pacp/8
2. J.A. Coderre, J.C.Turcotte, K.J.Riley et al., Technology in Cancer Research & Treatment 2 (5), 355 (2003)
Left to right:
Chemical structure of BPA, BSH, CuTCPH and boron-loaded liposome [Rolf F. Barth et al., Neurosurgery 44 (3), 433 (1999)].
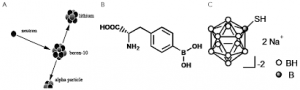
Neutron capture reaction of 10B
Currently available boron delivery agents: boronophenylalanine p-dihydroxyboryl-phenylalanine (BPA)
Currently available boron delivery agents: borocaptate sodium sulfhydryl borane Na2B12H11SH (BSH)
Sources:
http://www.thetcr.org/
www.com-info.org